Fall 2012
Dear No-Rosion Customer,
The summers just keep getting hotter. According to the National Oceanic and Atmospheric Administration, July was the hottest month on record for the contiguous U.S. since record keeping began in 1895. This only underscores the necessity of maintaining an optimally functioning cooling system.
But its not just a cooling system that prevents an engine from overheating. Its also the fuel system. Issues related to fuel quality, fuel octane, fuel delivery, the air/fuel mix, and combustion chamber cleanliness can all cause overheating, even in engines with cooling systems functioning at 100% efficiency!
The biggest single problem relates to the composition of todays fuel blends. Tetraethyl lead is the absolute best antiknock compound. Unfortunately its use in gasoline was banned by the EPA years ago. So in order to manufacture gasoline having sufficient antiknock qualities, or octane, refiners have had to resort to blending with oxygenates, most of which are either alcohols or ethers. Because of toxicity and cost issues associated with some oxygenates, the most popular one being used today is ethanol. Even when you think you are buying gasoline that contains no ethanol, these days referred to as E0, there is usually some ethanol in the blend.
In most states, ethanol is added, by law, to a minimum level of 5.9%. Most fuel pumps display a sticker stating the fuel may contain up to 10% ethanol, an intentional disparity which allows the minimum level to be raised over time without requiring modification of the labeling. Until late 2010, fuels retailers were only authorized to sell fuel containing up to 10% ethanol (E10). Then the EPA announced that this allowable limit would be increased to 15%, but only for vehicles built after 2007. Most new vehicle warranties (except for flexible fuel vehicles) authorize fuels that contain no more than 10%. So this is a point of contention between OEMS and the US government, and source of court challenges between the EPA, auto manufacturers, and oil companies.
Ethanol creates a multitude of problems in fuel systems, especially in carbureted engines built before 1990. From the factory, they are calibrated to run on one kind of fuel, and can not make adjustments like modern electronic fuel injection. Engines that were built before the introduction of ethanol were calibrated to run on straight gasoline. Ethanol contains extra oxygen, which throws off the air/fuel ratio, making the engine run too lean. Lean engines run hotter, and have drivability problems, such as hard starts and rough running.
Ethanol is hygroscopic. It readily and continuously absorbs water from humidity in air through a process known as emulsion. When ethanol becomes saturated, it separates from the gasoline, forming two separate solutions. This is called phase separation. An engine will not run on the (water-soaked) ethanol solution, which sinks to the bottom of the tank and is highly corrosive. It causes fuel tanks and lines to corrode from the inside out. Tiny pieces of corrosion byproducts break away from the corroded surfaces, and get lodged in carburetor inlets, needle valves, and jets. They can clog fuel filters, and generally wreak havoc to the entire fuel system. Emulsified gasoline is also less volatile, resulting in octane loss, hard starts, and rough/hot running.
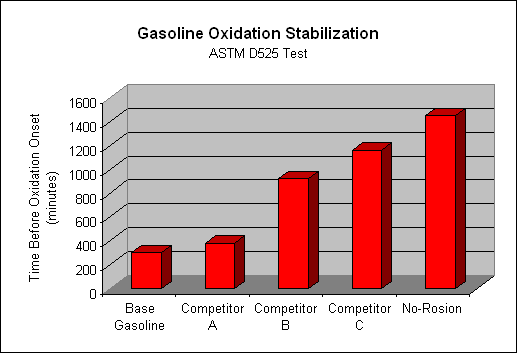
Then there is the issue of oxidation. Oxygen in air causes ethanol to oxidize, which forms insoluble, non-combustible gum byproducts. We have conducted extensive testing in this area, and found rapid onset of oxidation in all ethanol blends sold today. Simply put, todays blends are formulated to be burned today, not tomorrow! In some of our tests, we have seen a 20 gallon tank of gasoline completely oxidize in only 30 days!
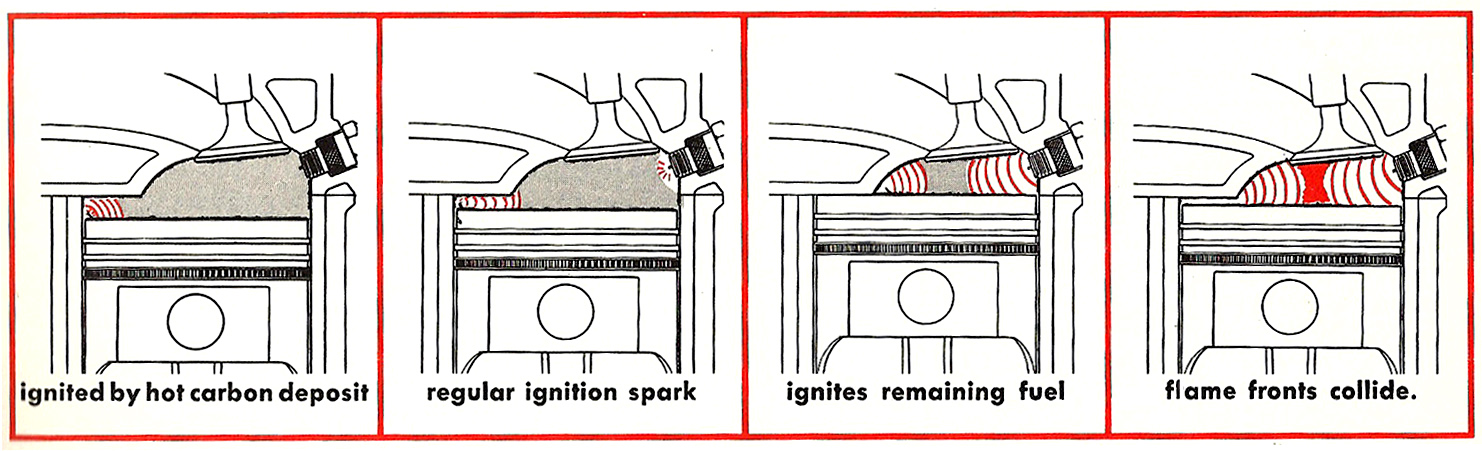
Running noncombustible byproducts of oxidized fuel through an engine causes carbon deposit buildup in the combustion chamber. Carbon deposits remain incandescent in the chamber, and cause preignition detonation.
Preignition is the igniting of the fuel charge before the regular ignition spark. If the premature combustion is completed before the occurrence of the regular spark, there may be no identifying noise. So you won’t even know that it is happening. However, if the regular ignition spark follows shortly after the preignition occurs, you will hear a tell-tale pinging noise when the two flame fronts collide. (As illustrated in the sequential series of images below.) Detonation produces significant additional cylinder heat, which can lead to engine overheating. It severely hinders engine power and performance, and increases the octane requirement of gasoline. In extreme cases, it can cause pistons to shatter, cylinders to burst, or cylinder heads to overheat and crack.
If you happen to have a fuel injected engine, oxidized gasoline also causes injector coking. Especially in todays new direct injection engines. This clogs and negatively impacts the optimal spray pattern of the injector. The result is poorly nebulized fuel, increased fuel consumption, and misfires. In severe situations, it can also cause the engine to run too lean, increasing operating temperature and the risk of catastrophic engine failure.
Plastic and rubber parts are susceptible to ethanols degradation byproducts as well. These include seals and O-rings in the fuel system and carburetors. Rubber materials tend to get hard and brittle with exposure, which can cause problems with needle valves in carburetors. Some of these rubber components can be partially dissolved with constant exposure to ethanol and its oxidation byproducts. Bits and pieces can be carried into the fuel system, causing clogs and misfires. Some older cars may still have plastic fuel filter bowls, which will degrade with exposure to ethanol and could leak.
Before 1990, many carburetors were built with alloys that are more prone to corrosion from ethanol. When ethanol contacts older alloy carburetor housings, corrosion can cause tiny orifices to clog. This results in hard starting and poor running, two of the most common complaints. This is one of the most serious problems for us old car owners, because there is usually no upgraded carburetor that can be retrofitted.
Now that you have been sufficiently warned of the pitfalls and hazards associated with todays fuel blends, there is only one piece of information left to provide: How to prevent them from occurring.
If you are a long-term customer, you will recall that from 1996 to 2006, we offered our high-quality No-Rosion fuel additive products. The chemistry in these products was based on hydrocarbon solvents, meaning that the cost to manufacture them was tied directly to petroleum prices. So when petroleum prices more than doubled from 2004 to 2006, so did our cost to manufacture these products. In 2006, we were faced with a difficult dilemma. Either double the price of our No-Rosion fuel additives, rendering them unaffordable, or reduce the cost by cutting quality. We did not find either of these two options to be acceptable. So we discontinued them.
It is our mission is to assist your maintenance efforts by providing only the absolute highest quality engine fluid additives available. As car guys ourselves, we will not manufacture any product that we do not use in our own cars. But discontinuing these products was difficult, because we are well aware of the need for such products.
Many of you called and asked if there was any product available that would perform similar to our No-Rosion Combustion Optimizer. We recommended Chevron Techron product. Even though it does not include the fuel stabilization performance of our product, it does contain some pretty good detergent chemistry. So it was probably the best replacement product available. That is, until now.
We are happy to announce that No-Rosion Fuel System Combustion Optimizer is back, and better than ever!
Significant advances in fuel additive chemistry prompted us to initiate research and development of our new fuel additives a few years ago. No-Rosion Combustion Optimizer is now 100% synthetic, and utilizes an aliphatic isoalkane solvent. It contains an entirely new, proprietary polyether amine (PEA) detergent. The highly-concentrated nature of PEA allows it to be packaged in a more compact, half-pint bottle. Packaging in a more compact bottle effectively cuts in half the amount of solvent necessary as a carrier. This, in turn, results in a more reasonably priced final product. Best of all, it drastically outperforms our old formula in its ability to prevent all of the fuel system issues referenced on the previous two pages. HOW??
First and foremost, it contains ingredients that stabilize gasoline. These ingredients prevent ethanol and other oxygenates in gasoline from oxidizing to form insoluble gums that cause carbon deposits and coking.

No-Rosion Combustion Optimizer also contains ingredients that protect against emulsion, and inhibit corrosion of all metals in a fuel system. Through utilization of dispersants, phase-separated ethanol in fuel is solubilized and burned through the combustion process – without associated octane loss or risk of deposit formation.
So the product effectively stabilizes fuel, thus preventing deposit buildup and associated problems to fuel systems and engines. But what about engines in which there are pre-existing carbon deposits from having already run broken-down fuel? That is where the PEA detergent technology comes into play.
The PEA in our product fully dissolves carbon deposits in combustion chambers and on intake valves. It washes them away from the surface, allowing them to be fully burned during the combustion process. It also very effectively removes gums and varnishes formed inside fuel tanks, fuel lines, carburetors, and injectors. Its presence even allows already broken-down fuel to be safely burned without the risk of forming deposits.
No-Rosion Combustion Optimizer is the only product available that provides the three most important performance features, all in one product: (1) clean-up, (2) keep-clean, and (3) fuel stabilization. One concentrated half-pint bottle treats 20 gallons of fuel. It treats any type or grade of fuel, will not harm catalytic converters or oxygen sensors, and is EPA, CARB, and TOP-TIER compliant. Add it to your tank before and after winter storage for fuel stabilization, and every 3,000 miles of driving to maintain a clean fuel system.
The absence of tetraethyl lead from todays fuel blends creates more problems that those already mentioned. It also drastically reduces the octane level of fuel, and can cause recession of non-hardened valve seats in the engines of our old cars. That is why we are also re-introducing our No-Rosion Fuel System Octane Booster!
As you may already know, many octane boost products on the market today utilize oxygenate ingredients, such as ethanol. This means that they can cause all the same problems already mentioned. Ours is completely different. It contains the metallic ingredient, methylcyclopentadienyl manganese tricarbonyl (MMT) in a high flash point aromatic solvent. MMT is widely recognized as the nearest equivalent to tetraethyl lead, and is the only metallic octane boost ingredient legally available today for use in street-driven vehicles.


But it is not just engines of older cars that benefit from the MMT in our No-Rosion Octane Booster. MMT also happens to be an excellent phosphorous scavenger. Why is this significant? Because many engine oils today contain phosphorous as an anti-wear ingredient. During combustion, trace amounts of phosphorus from the crankcase make their way into exhaust gases. Phosphorus builds up on catalyst surfaces, causing a reduction in catalyst efficiency, and increased emissions. The proprietary blend of MMT in our formula removes phosphorus in catalytic converters. Over time, this restores catalyst efficiency, and reduces emissions.
Street-driven vehicles add one concentrated half-pint bottle to 20 gallons of gasoline to boost octane by 10 points (1 number). Off-road and race vehicles add two bottles to 20 gallons to boost octane by 20 points (2 numbers). Non-catalyst off-road and race vehicles can add four bottles to 20 gallons to boost octane by 40 points (4 numbers). Note: Adding more than one bottle to 20 gallons of gasoline is not EPA street-legal.
Using both of our fuel additives at the same time does provide synergistic performance enhancements. This includes the passing of emissions tests for vehicles that had previously failed them.
As the engine fluid maintenance needs for our old cars continue to evolve, so too does our product offering. This is why we now offer high quality No-Rosion additives for both your cooling system and fuel system.
Thank you for being a customer. We appreciate your business, and look forward to continuing to be of service.
Sincerely,
Applied Chemical Specialties, Inc.